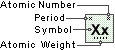Iodine
Symbol
I
Atomic Number
53
Atomic Weight
126.90447
Element Classification
Discovered By
Bernard Courtois
Discovery Date
1811 (France)
Name Origin
Greek: iodes (violet colored).
Density(g/cc)
4.93
Melting Point
386.7
Boiling Point(K)
457.5
Appearance
Shiny, black nonmetallic solid
Atomic Radius(pm)
n/a
Atomic Volume(cc/mol)
25.7
Covalent Radius(pm)
133
Ionic Radius
50 (+7e) 220 (-1e)
Specific Heat
0.427 (I-I)
Fusion Heat
15.52 (I-I)
Evaporation Heat(kj/mol)
41.95 (I-I)
Thermal Conductivity(@25°C W/m K)
(0.45)
Debye Temperature
n/a
Pauling Negativity Number
2.66
First Ionizing Energy(kj/mol)
1008.3
Oxidation States
7, 5, 1, -1
Electronic Configuration
[Kr] 4d10 5s2 5p5
Lattice Structure
Orthorhombic(ORC)
Lattice Constant
7.720
Lattice C/A Ratio
n/a