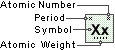Plutonium
Symbol
Pu
Atomic Number
94
Atomic Weight
244.0642
Element Classification
Discovered By
G.T.Seaborg, J.W.Kennedy, E.M.McMillan, A.C.Wohl
Discovery Date
1940 (United States)
Name Origin
Named for the planet Pluto.
Density(g/cc)
19.84
Melting Point
914
Boiling Point(K)
3505
Appearance
Silvery-white, radioactive metal
Atomic Radius(pm)
151
Atomic Volume(cc/mol)
n/a
Covalent Radius(pm)
n/a
Ionic Radius
93 (+4e) 108 (+3e)
Specific Heat
n/a
Fusion Heat
2.8
Evaporation Heat(kj/mol)
343.5
Thermal Conductivity(@25°C W/m K)
(6.7)
Debye Temperature
n/a
Pauling Negativity Number
1.28
First Ionizing Energy(kj/mol)
491.9
Oxidation States
6, 5, 4, 3
Electronic Configuration
[Rn] 5f6 7s2
Lattice Structure
Monoclinic(MCL)
Lattice Constant
n/a
Lattice C/A Ratio
n/a