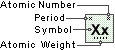Zinc
Symbol
Zn
Atomic Number
30
Atomic Weight
65.39
Element Classification
Discovered By
Known to the ancients.
Discovery Date
n/a (Germany)
Name Origin
German: zink (German for tin).
Density(g/cc)
7.133
Melting Point
692.73
Boiling Point(K)
1180
Appearance
Bluish-silver, ductile metal
Atomic Radius(pm)
138
Atomic Volume(cc/mol)
9.2
Covalent Radius(pm)
125
Ionic Radius
74 (+2e)
Specific Heat
0.388
Fusion Heat
7.28
Evaporation Heat(kj/mol)
114.8
Thermal Conductivity(@25°C W/m K)
116
Debye Temperature
234.00
Pauling Negativity Number
1.65
First Ionizing Energy(kj/mol)
905.8
Oxidation States
2
Electronic Configuration
[Ar] 3d10 4s2
Lattice Structure
Hexagonal(HEX)
Lattice Constant
2.660
Lattice C/A Ratio
n/a